Tirzepatide 15mg represents a significant advancement in peptide research, serving as a dual agonist of both glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors. This innovative compound combines two insulinotropic effects within a single molecular structure, making it particularly valuable for metabolic research applications.



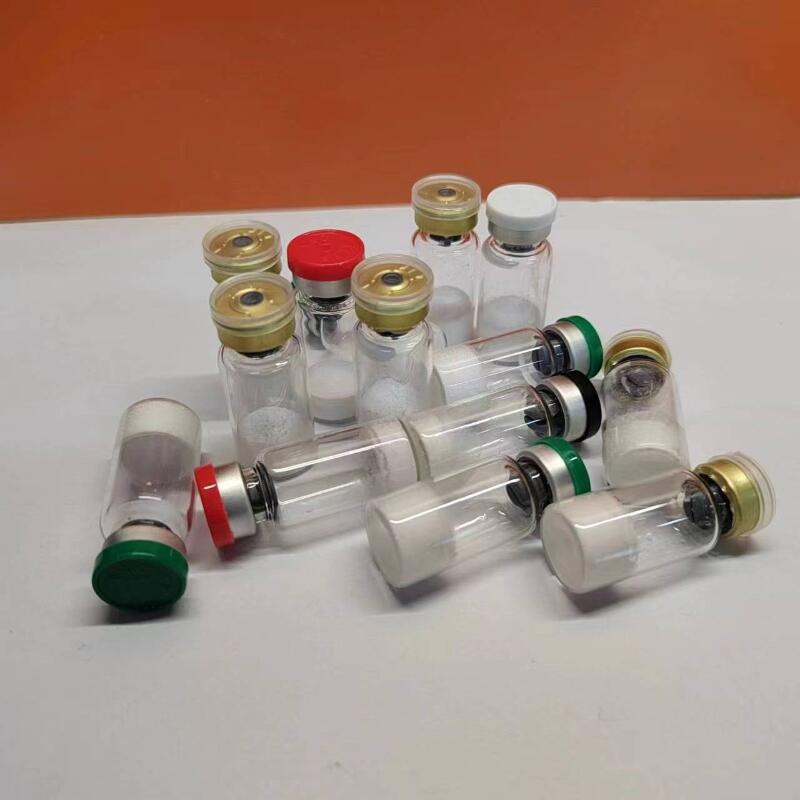

The tirzepatide 15mg formulation appears as a white lyophilized powder requiring reconstitution before use. Proper storage after reconstitution is essential, with recommended temperatures between 2°C and 8°C to maintain stability and efficacy. The compound's molecular structure (C225H348N48O68) and weight (4813.527g/mol) contribute to its unique pharmacological properties.
Research applications for tirzepatide 15mg primarily focus on metabolic studies and weight management research. The dual receptor activation mechanism provides researchers with a valuable tool for investigating glucose metabolism and insulin secretion pathways. The availability of tirzepatide 15mg in various concentrations allows for flexible experimental design across different research protocols.
Quality assurance is paramount in our manufacturing process. Each batch of tirzepatide 15mg undergoes rigorous testing to ensure purity levels exceed 99.0%. Our production facilities maintain strict quality control measures throughout the manufacturing process, from raw material selection to final packaging. The tirzepatide 15mg product is packaged in sterile vials with multiple size options available to accommodate various research requirements.
Customization options include various packaging configurations and concentration levels. Research institutions can request specific formulations of tirzepatide 15mg to meet their unique study parameters. Our technical support team provides comprehensive assistance regarding proper handling, storage, and application protocols for tirzepatide 15mg in research settings.
This product is supplied exclusively for research purposes and laboratory use. Proper safety protocols must be followed when handling tirzepatide 15mg, including appropriate personal protective equipment and laboratory conditions. Researchers should consult relevant literature and institutional guidelines before incorporating tirzepatide 15mg into their studies.